|
3 likes
comment
share |
4 min reading time
Simply a gift for you today. If you have anything to do with medical device quality systems, click here for FDA slides for immediate download. Four slides that stood out to meIt was hard to narrow down the four hours from four presentations to four slides. But here goes, from me to you. 😊
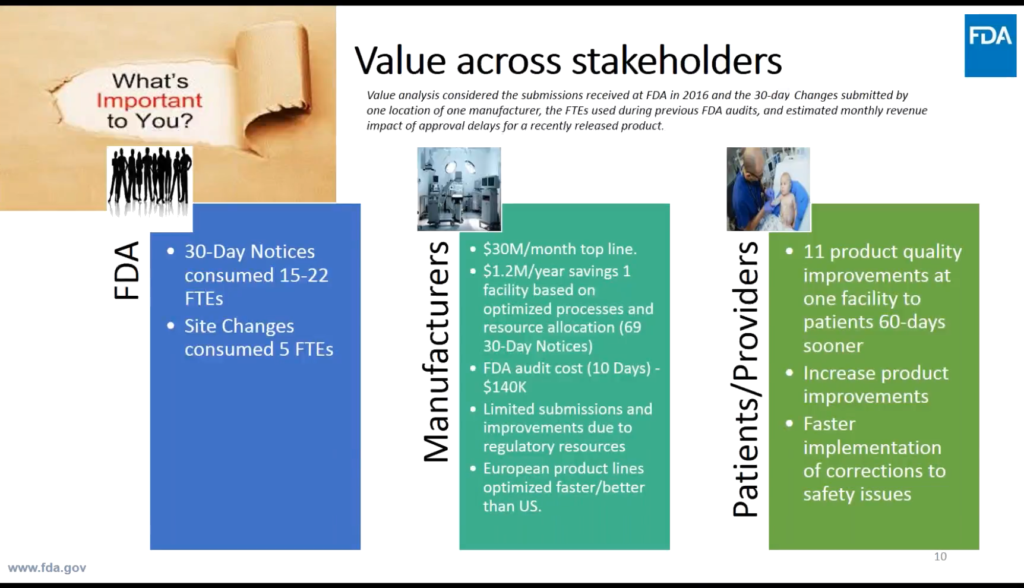
Above: FDA Case for Quality Program Manager Francisco Vicenty explained why they’re leading the Initiative, saying, “We are in this together: The Agency, the medical device manufacturers, the providers, the hospitals [to] drive the best public health outcomes… We need to change from a compliance mindset to a drive on quality.” The slide shows the present economic impact of today’s FDA processes. “People stopped submitting ideas or change activity.”
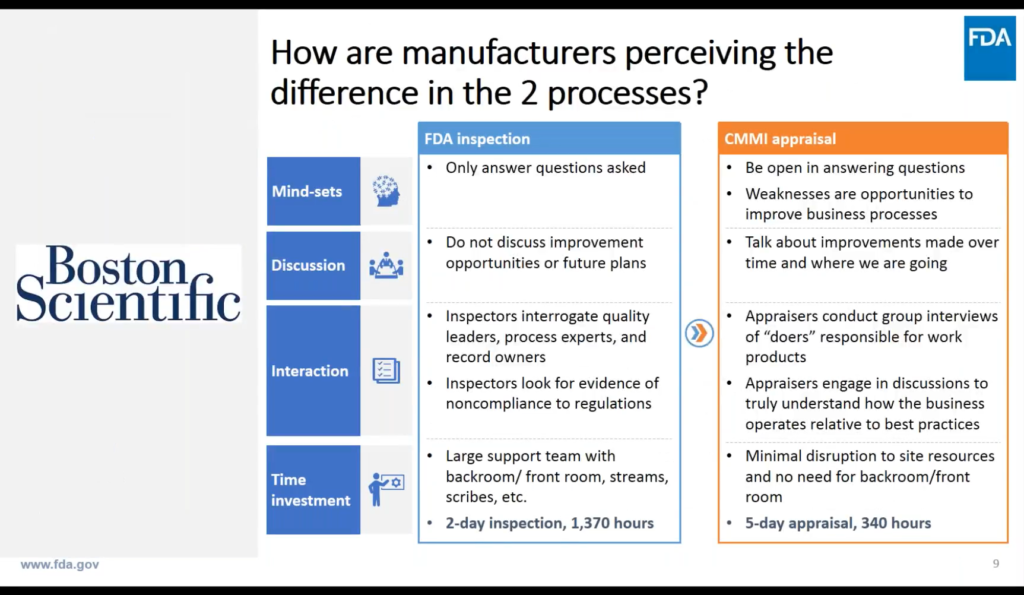
Above: This is a slide Francisco “stole” from Boston Scientific, how they see the difference between an FDA inspection and the new approach, using Capability Maturity Model Integration (CMMI).
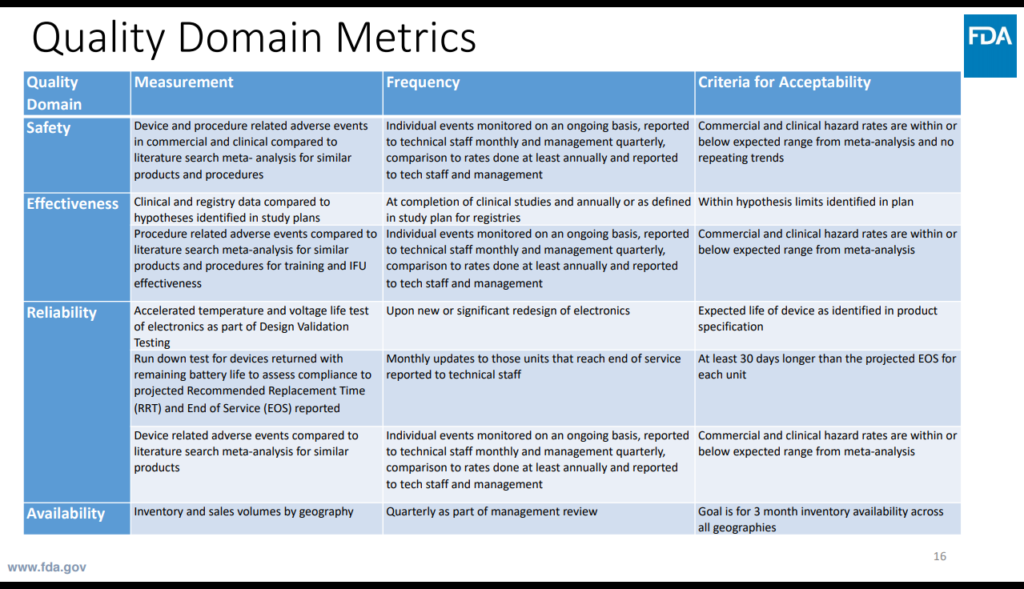
Above: A medical device manufacturer proposed this slide. Francisco found it “exceedingly helpful,” giving FDA areas where the manufacturer felt their metrics aligned with the Case for Quality Initiative: How they monitor the safety, effectiveness, reliability, and availability of their product.
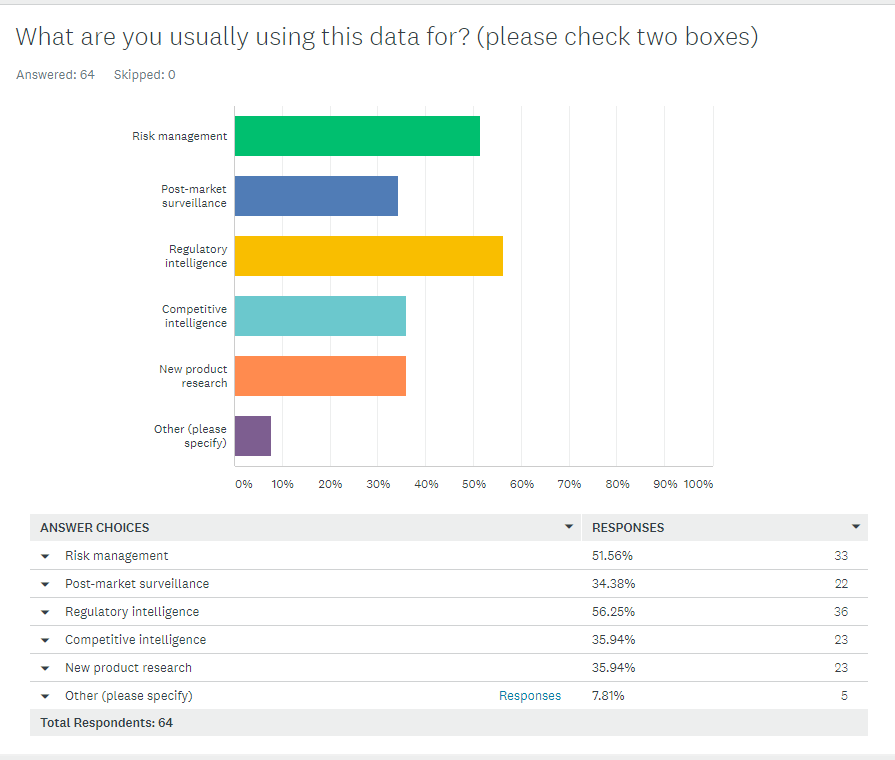
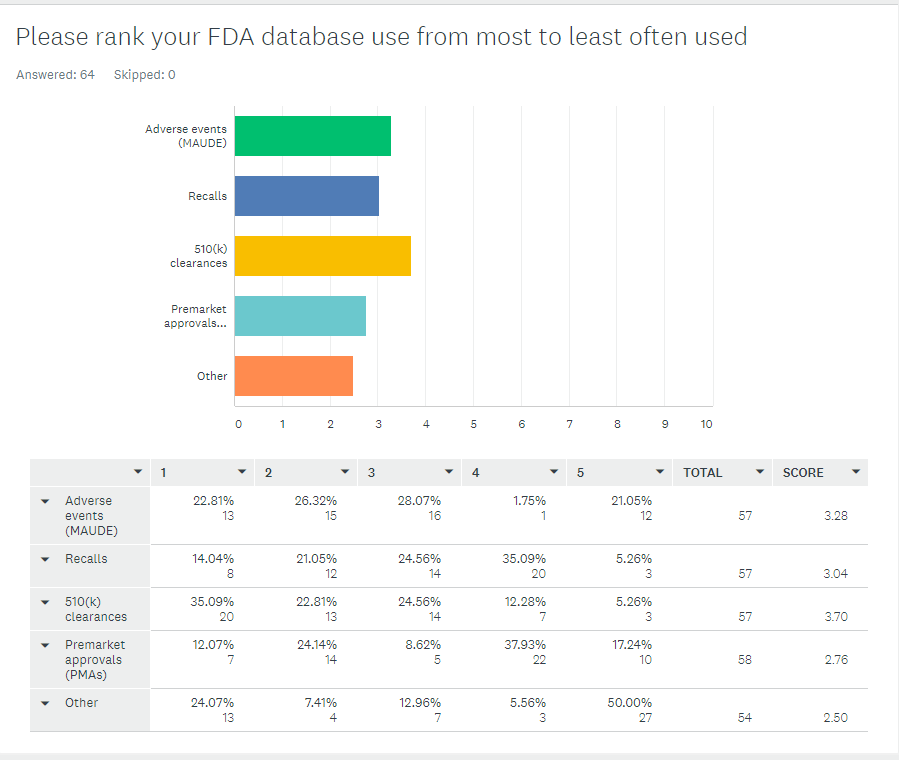
Above: The main obstacle for medical device manufacturers to justify technology investments? The computer system validation effort: The regulatory and compliance risk associated with implementing that system. Sometimes the cost was 1.5 to 2 times the base system cost, a “huge eye-opening moment for the FDA.” Download the slides, listen to the recordings.I’ll close this section as I began it. If you have anything to do with medical device quality systems, click here for FDA slides for immediate download. And thanks to Greenlight Guru, the only Quality Management Software designed specifically for the medical device industry, for sponsoring the presentations. +++ An easier way to use FDA’s databasesI’m hosting a webinar next week on how to dramatically simplify extracting data out of FDA databases. You can click the yellow button below to join me. This is interesting. So, among 64 people who took the survey, this is what they said about their FDA data use. I wonder how you compare.
I hope you’ll join us for the live event on December 11 at 2 p.m. Eastern Daylight Time. Click here to register: The slides and replay will be available to all who register. +++ Leave a penny, take a pennyI had a live chat with Maggie Holland from Sweden this morning. She was following up on her question about transitioning to MDR.
Please tell your friends about the site and get them registered and involved. This only works if the whole medical device ecosystem comes together and participates in the resource. I don’t know the answers all by myself! It’s like the old “leave-a-penny, take-a-penny” containers you’d see at checkouts. I’m hoping you’ll give some pennies back, in the form of expanding our community (for example, share this email with your department), or contributing questions and answers to our community page. Plus, it makes a lovely holiday gift 🎄 for Joe Hage! So thank you in advance. +++ Inquiring Minds Want to Know!• Maggie’s question about transitioning to MDR and avoiding clinical equivalence. • Whitney’s question about investigational use labels. • Lena’s question about the impact of Netflix on our industry. • Robyn’s fears about the CRISPR twins! • José’s question about MDR2017/745. (Hmm. Two MDR questions! We covered MDR recently.) +++ Thank you for being part of our Medical Devices Group community!If you’re looking for work, check out the newly posted jobs here! Make it a great week.
Joe Hage P.S. I’m excited about our upcoming 10x keynote presentation. Former Proove Biosciences CEO Brian Meshkin will answer the question he gets so often, “What on Earth Happened at Proove Biosciences?!“ Marked as spam
|
Meet your next client here. Join our medical devices group community.
We still use LinkedIn to access our site because it’s the only way to “pull in” your LinkedIn photo, name, and hyperlink to your profile page, all vital in building your professional network. When you log in using LinkedIn, you are giving LinkedIn your password, not me. I never see nor store your LinkedIn credentials.



 I told her I’d raise it here today, then asked her for a favor. I realize now it’s a favor I should ask of every reader.
I told her I’d raise it here today, then asked her for a favor. I realize now it’s a favor I should ask of every reader.