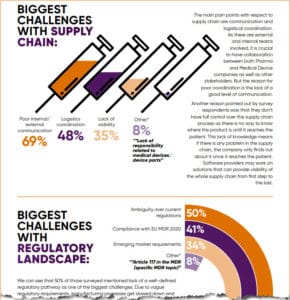
|
2 likes
6 comments
share |
3 min reading time
God bless Michael Seres. He was diagnosed with Crohn’s disease, an incurable bowel condition, at age 12. His first surgery at 14, he’s 25 further surgeries stronger, having weathered intestinal failure and two bouts of cancer. He’s now awaiting a stem cell transplant. So what does Michael do with all his free time? As a patient, he says,
The Ostomy Market: Size, Problems, and Michael’s SolutionMichael wove a deeply personal story into his narrative. I heartily encourage you to learn more, here presented in a beautifully edited presentation. His slides and transcript are also available for you now. Did you know?
The 11Health Solution11Health added sensors and technology in ostomy bags to measure real-time output. Doing so gives you early warnings of leaks, spills, infection markers and more. It’s quite genius, actually, added your humble editor. If you’re interested in the ostomy market, as a patient, future employee, investor, or strategic partner, you’re going to want to access the replay, slides, and transcript. Michael, congratulations. I’m honoured (the “u” in your honour) to call you my friend. 🤗 The Combination Products Market
The report covers medical device and pharma company cases on managing combination products through development, implementation, launch, and post-market surveillance. Related: This year’s Combination Products Forum will be 22-24 October 2019 in Berlin, Germany. So what else?No matter what story I told, my mother-in-law’s next question would be, “So, what else?” Here’s “what else,” Mom:
Thank you for being part of our Medical Devices Group community!Please Make it a great week.
Joe Hage P.S. Group member Susan Hartman wrote me, “The suggested ‘get around’ for MDR will not work for the UK, at least. NHS announced it will not accept product that is not MDR compliant from April 2020 onward. We’re learning as we go, Folks! Here’s the original discussion about the MDR workaround. Marked as spam
|
Meet your next client here. Join our medical devices group community.

Private answer
Joe Hage
from Jeff Nichols Marked as spam
|
|

Private answer
Joe Hage
from Mark Gordon Cooper Marked as spam
|
|

Private answer
Joe Hage
from Ellen Coolidge Marked as spam
|
|

Private answer
Joe Hage
from Chen Sirkis Marked as spam
|
We still use LinkedIn to access our site because it’s the only way to “pull in” your LinkedIn photo, name, and hyperlink to your profile page, all vital in building your professional network. When you log in using LinkedIn, you are giving LinkedIn your password, not me. I never see nor store your LinkedIn credentials.